Selecting appropriate chemical protective clothing and ensuring its proper use are important responsibilities of every employer. Standard methods, practices, guidelines, performance criteria and evaluations of hazardous situations in the workplace can help employers make informed decisions. However, these decisions can be complicated when mixtures are involved or when temperatures vary. That’s because additional issues must be considered, including:
• How does permeation change when chemicals are mixed together?
• On which chemical should permeation be measured?
• How does permeation change when the makeup of the mixture is changed?
• How does permeation change when temperature changes?
Before attempting to address these issues, a review of permeation might be useful. Simply stated, permeation involves:
• Absorption of the chemical through the outer surface;
• Build-up of the chemical in the fabric; and
• Evaporation of the chemical from the inner surface.
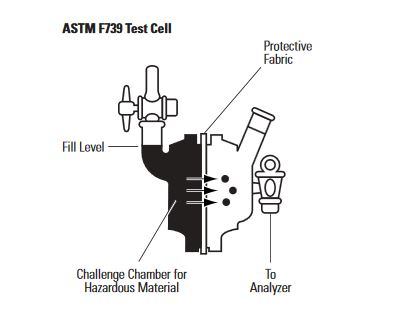
The standard method used in North America for measuring the permeation of chemical protective apparel material is ASTM E739 – “Standard Test Method for Resistance of Protective Clothing Materials to Permeation by Liquids or Gases under Conditions of Continuous Contact.” In this test, the material is clamped between two chambers. One chamber is filled with the challenge chemical. The other chamber is checked for the presence of the challenge chemical. The amount of chemical in the sample chamber is measured over time to determine the breakthrough time and permeation rate.
Mixtures
Each combination of barrier and chemical has a characteristic breakthrough time and permeation rate. This unique relationship is due to a combination of physical and chemical interactions. Mixtures often have chemical and physical characteristics that are different from those of their components. Take the classic mixture known as “aqua regia” (royal water), for example. Aqua regia is a mixture of two mineral acids. Individually, neither will dissolve gold; but in a specific ratio, they will.
Because mixtures have unique chemical and physical characteristics, it is reasonable to expect that the permeation of the mixtures will be different from the permeation of the components. Some differences can be explained. For example, some rubber-like barrier materials swell when exposed to certain solvents. This swelling is a change in the physical structure of the barrier. If there are other chemicals present, the permeation might be different because the physical structure of the barrier has changed.
The individual chemicals in a mixture may permeate at different rates. Thus, the mixture in the sampling chamber will be different from the mixture in the challenge chamber. Measuring total mass in the sample chamber will not provide information on the permeation of the individual chemicals. With mixtures, either certain assumptions must be used or each component must be measured.
There are a number of ways to measure chemicals that permeate. Few of these techniques “see” only one chemical. These techniques work well when measuring the permeation of individual chemicals. However, with mixtures, it is difficult to know whether you are seeing a small amount of an easy-to-measure chemical or a large amount of a hard-to-measure chemical.
Ideally, the analysis should be able to distinguish between the components in the mixture. The more chemicals present, the more sophisticated the separation technology. In addition, checks must be made to ensure that one chemical does not interfere with the detection of another.
Another important point to remember is that mixtures found in industry seldom have fixed proportions. One example is black liquor, a mixture commonly encountered in the kraft paper industry. Black liquor is a caustic mixture of sodium sulfides, sulfites, sulfates and hydroxide, combined with dissolved lignin, rosins, tall oils and turpenes. The organic components of this mixture change substantially with the type of wood used. What’s more the inorganic contents are different at each paper mill as a result of changes in process equipment and processing conditions. Although a general understanding of the permeation behavior of black liquor components can be developed, unexpected variations in composition might lead to unexpected permeation behavior.
In summary, when dealing with mixtures, it is best to measure permeation using the mixture of concern. Permeation of each harmful component should be determined separately. If the components are similar, certain conservative assumptions can be applied. If the components are dissimilar, then sophisticated standardization and separation techniques may be necessary. In either case, obtaining knowledge of the chemistry of the components, the performance of the barrier in similar situations and a conservative selection of materials is the first place to start.
How Temperature Affects Permeation
In general, permeation is expected to increase with increasing temperatures. However, there are exceptions to this generality. The temperature response of permeation rates depends on the barrier material and the chemical. In general, permeation rate increases by a factor of 1.2 to 3.5 (depending on the material and the chemical) for each 5.60 C (10°F) increase in the surrounding temperature. For example, at room temperature, a 6°C increase in temperature will increase the permeation rate of simple gases through an oriented polyester film from 1.3 to 2.1 fold, depending on the gases. (“Diffusion in Polymers”, J. Crank and G.S. Park, Ed., Academic Press, 1968, PP 46-50). This illustrates the behavior of one film and a few chemicals within a narrow temperature range.
The effect of temperature on breakthrough times will diminish with temperature, but not with the same impact as seen on permeation rates.
This information is based upon technical data that DuPont believes to be reliable. It is subject to revision as additional knowledge and experience are gained. DuPont makes no guarantee of results and assumes no obligation or liability in connection with this information.
It is the user’s responsibility to determine the level of toxicity and the proper personal protective equipment needed. The information set forth herein reflects laboratory performance of fabrics, not complete garments, under controlled conditions. It is intended for use by persons having technical skill for evaluation under their specific end-use conditions, at their own discretion and risk.
Anyone intending to use this information should first verify that the garment selected is suitable for the intended use. In many cases, seams and closures have shorter breakthrough times and higher permeation rates than the fabric. If fabric becomes torn, abraded or punctured, end user should discontinue use of garment to avoid potential exposure to chemical. SINCE CONDITIONS OF USE ARE OUTSIDE OUR CONTROL, WE MAKE NO WARRANTIES, EXPRESSED OR IMPLIED, INCLUDING, WITHOUT LIMITATION, NO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE AND ASSUME NO LIABILITY WHATSOEVER IN CONNECTION WITH ANY USE OF THIS INFORMATION.
This information is not intended as a license to operate under or a recommendation to infringe any patent or technical information of DuPont or others covering any material or its use.
Brought to you by DuPont
DuPont Personal Protection's product lines include protective and performance solutions that keep industrial workers safe and your operations running smoothly. DuPont PPE is dedicated to keeping you protected—every step of the way. DuPont Personal Protection product lines range from disposable clothing to work clothing.

Related Articles
![Why the Cheapest Safety Gloves Aren’t Always the Smartest [Infographic]](https://images.ctfassets.net/5j4ln2up7bt7/2gVEyRZylkBIlvTDtTRRc7/dde0c00e4846d6a88d56a7a68f09332a/mcr-PD5931_action4571-thumb.jpg)
Why the Cheapest Safety Gloves Aren’t Always the Smartest [Infographic]

Why Mental Health Belongs in Every Safety Conversation

Microfiber Tech: MFT PRO Gloves Pair Comfort and Performance

Reliable Secondary FR Gear for Chemical Protection


